eIFU – Electronic Instructions for Medical Devices
eIFU, in addition to being the industry acronym (Electronic Instruction For Use), is the name we have given to the our web platform That eliminates the need for paper sheets inside the products to facilitate consultation by professionals and improve your business processes.
Download the brochure Request a demo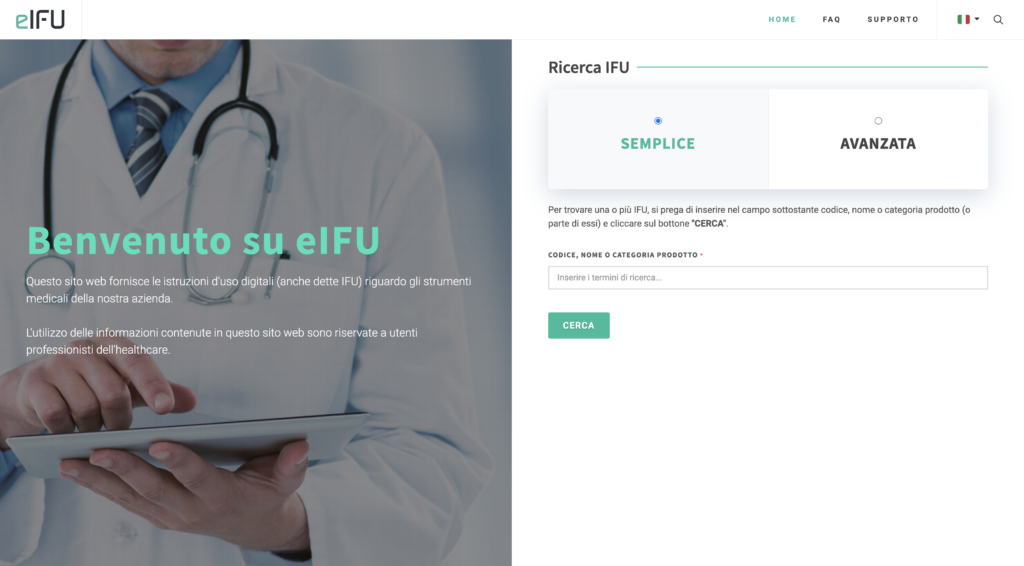
The market context and management of IFUs
The evolution of instruction sheet
Medical device manufacturing companies are required to provide IFU (Instructions For Use) in order to ensure correct and safe use of their products.
According to current regulations, for example EU Regulation 207/2012 and FDA Blue Book, this information can be made available in digital format, for example through a web portal.
Discover the advantages for companies in the medical sector
At RealBit we have been working for years in the creation of software dedicated to the medical market. By adopting our innovative solution you will obtain tangible advantages in terms of cost/time savings and updating of documentation to which add logistics optimizations and a concrete contribution to environmental sustainability.
Save costs
and time
With eIFU, instructions are digitized and at your fingertips, wherever you are and on any navigation device.
Timely updates
Your manuals will always be updated with the latest adjustments resulting from regulations and legislative obligations.
Optimize warehouse and products
No more searching for paper manuals, you will always have updated information without demanding logistical interventions.
Contribution to sustainability
By choosing eIFU, you contribute to a greener future by eliminating the need for printed materials.
HOW DOES IT WORK

Simple, Intuitive, Convenient
With eIFU, IFU management is safe and rapid.
Just a few clicks and the new revisions of your IFU will be online available to your customers.
Through QR codes or simple searches, your customers will quickly have the latest version of the correct IFU for the product used.
Electronic Instructions VS Paper
Thanks to eIFU's live monitoring system, you have constant control over users. Generate useful reports and statistics compliant with 21CFR11 to ensure complete traceability and compliance with current regulations.
01. Real-time traceability
02. Multi-language, multi-user, multi-device
03. Highly configurable
04. Available in CLOUD and OnPREMISE
05. Including validation documents
06. Can be integrated with company management systems
Legal Compliance and Certifications
Compliant with the law and always updated: eIFU is designed in compliance with the latest EU and FDA regulations. Our commitment to ensuring legal compliance means that instructions for medical devices will always be in line with the latest regulatory provisions. Furthermore, eIFU frees you from annoying and time-consuming validation procedures, offering you validation documentation in full compliance with current laws.
Legal Certification: Thanks to our in-depth knowledge of official directives, we guarantee you a pre-validated site free of problems with notified bodies. Our validation ensures that eIFU meets rigorous quality and safety standards. You can trust a product that meets the highest standards, giving you the peace of mind you deserve.
